JOHANNES EISELE/AFP via Getty Images
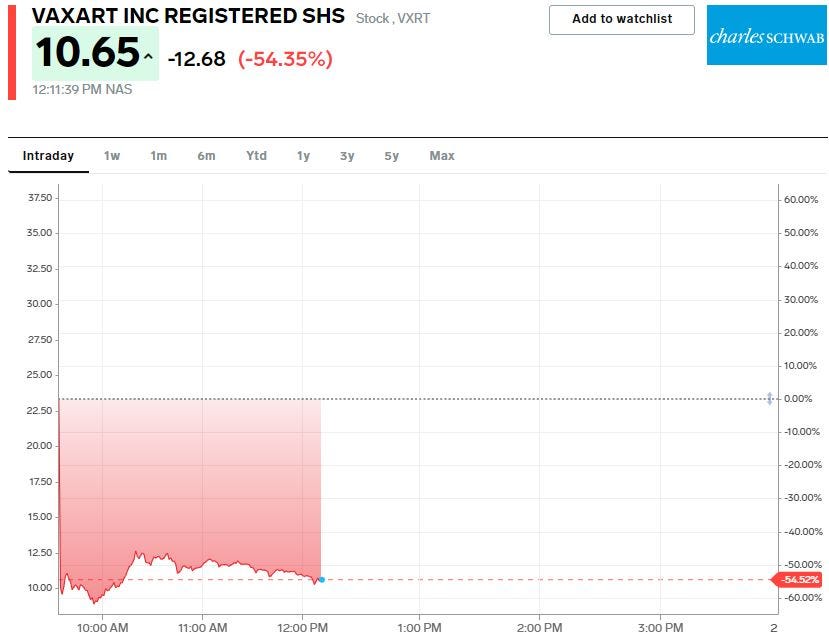
- Vaxart crashed as much as 62% on Wednesday after the company said its oral COVID-19 vaccine candidate did not produce antibodies.
- The results came from a preliminary data analysis of its Phase 1 trial of VXA-CoV2-1.
- Sign up here for our daily newsletter, 10 Things Before the Opening Bell.
Vaxart said it observed positive preliminary data from its phase 1 clinical trial of its oral COVID-19 vaccine candidate, but investors weren’t buying it.
Shares of Vaxart crashed as much as 62% on Wednesday after the company said its VXA-CoV2-1 vaccine candidate did not product antibodies, which indicates little to no immune response to the vaccine among patients.
“Neutralizing antibodies were not detected in serum and IgG responses were not detected in most subjects,” Vaxart said in a press release.
The clinical stage biotechnology company said the vaccine, which is delivered in the form of a tablet, was generally well-tolerated, with no severe adverse events reported.
Vaxart said the study did reach primary and secondary endpoints related to safety and immunogenicity.
The company said last year that it was selected by the US government to be included in Operation Warp Speed, which is a multi-billion dollar program designed to accelerate the development and administration of successful COVID-19 vaccines. That announcement may have been an overstatement.
Vaxart said in a SEC filing late last year that the firm is under federal investigation for exaggerating its involvement in Operation Warp Speed. In response to the investigation, the company has said that the statements made in its original press release were accurate and "any allegation to the contrary is baseless."